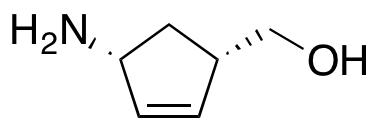
(1S,4R)-4-Amino-2-cyclopentene-1-methanol
| Product Name |
(1S,4R)-4-Amino-2-cyclopentene-1-methanol
|
| Product Code |
S00007554
|
| Chemical name |
(1S,4R)-4-Amino-2-cyclopentene-1-methanol
|
| Synonyms |
(1S,4R)-4-Amino-2-cyclopentene-1-methanol;
|
| Impurity |
|
| CAS Number |
136522-35-5
|
| Alternate CAS # |
|
| Molecular form |
C₆H₁₁NO
|
| Appearance |
|
| Melting Point |
|
| Mol. Weight |
113.16
|
| Storage |
|
| Solubility |
|
| Stability |
|
| Category |
Chiral Reagents, Intermediates, Nucleotides, Bases & Related Reagents
|
| Boiling Point |
|
| Applications |
(1S,4R)-4-Amino-2-cyclopentene-1-methanol is a reagent for preparing cyclic ADP-carbocyclic-ribose analogs via Yoshikawa’s phosphorylation and cyclocondensation (1). (1S,4R)-4-Amino-2-cyclopentene-1-methanol is also an intermediate for the synthesis of Abacavir (A105000). Abacavir is a carbocyclic 2’-deoxyguanosine nucleoside reverse transcriptase inhibitor and an anti-HIV drug used to treat HIV infection (2). Intracellular enzymes convert Abacavir to its active form, carbovir-triphosphate (CBV-TP), which then selectively inhibits HIV reverse transcriptase by incorporating into viral DNA (3). Abacavir is metabolized in the liver by uridine diphosphate glucuronyltransferase and alcohol dehydrogenase resulting in inactive glucuronide and carboxylate metabolites, respectively.
|
| Dangerous Goods Info |
|
| References |
(1) Kudoh, T., et al.: Nucleosides Nucleotides Nucleic Acids 24, 655 (2005)
(2) Jackson, A., et al.: Antivir Ther. 17, 19 (2012)��
(3) Yuen, G. J., et al.: Clin Pharmacokinet. 47, 351 (2008)�
|
| Extra Notes |
|
| Documents (MSDS) |
No Data Available
|
| Keywords |
Buy 136522-35-5 | Purchase 136522-35-5 | Order 136522-35-5 | 136522-35-5 supplier | 136522-35-5 manufacturer | 136522-35-5 distributor | 136522-35-5 cost |